Background: Our group recently reported, in a prospective multicentre phase 2 study of 31 patients with higher-risk (HR)-MDS or CMML receiving CPX-351 as first-line induction treatment 87% of overall responses (including 64.5% of CR/ CR according to ELN2017 criteria), and 93.5% of the patients could be bridged to transplant (GFM-CPX MDS trial, Peterlin et al. Lancet Haematol 2023). We had also previously reported, in AML, that the soluble forms of FLT3 ligand (FL) and IL6 could be prognostic factors of refractoriness to chemotherapy and overall survival (OS) (Peterlin et al. Cancer Medicine, 2021). A secondary objective of the GFM-CPX MDS trial was to assess the prognostic value of these two cytokines in HR-MDS/CMML receiving CPX-351.
Methods: All patients included in the GFM-CPX MDS trial (clinicaltrials.gov#NCT04273802), induction chemotherapy with CPX-351 (44 mg Daunorubicin/100 mg Aracytine/m 2 on days 1, 3, and 5) also consented for the present biological FLAM-MDS sub-study. Using a new cytokine multiplex assay from Milliplex (MILLIPLEX®), FL levels (expressed in pg/mL) were evaluated on Day (D) 1, D8, D15 and D22 of the first cycle of induction; IL-6 (expressed in pg/mL) and CRP (known to be correlated with IL6, expressed in mg/L, normal value 0-5) levels were evaluated on D1 and D22 only. Patients who failed to achieve response (CR or CRi or MLFS, ELN2017 criteria) after the first induction cycle could receive a second one. The impact on CR/CRi rate after one cycle of CPX-351, OS and EFS of FL, IL-6 and CRP dosages and their kinetic profile during this first cycle were studied.
Results: Dosages could be performed in 28 of the 31 patients included ( Table 1). Overall response (CR+CRi+MLFS) after cycle 1 was achieved in 24 (87%, MLFS n=7, CR+CRi n=17) patients; 3/4 non-responders received a second induction (1 achieved CR). Allograft was performed in 26/28 (93%). With a median follow-up of 18 months for surviving patients, 1y and 18-month OS were 79% and 75%, respectively. 1y and 18-month EFS were both 76%, respectively. Achieving CR/CRi versus MLFS/non-response was associated with better 1-y EFS (71% (52-96) vs 27% (10-72), p=0.009) but not 1-y OS (82% (66-100) vs 73% (51-100), p=0.8).
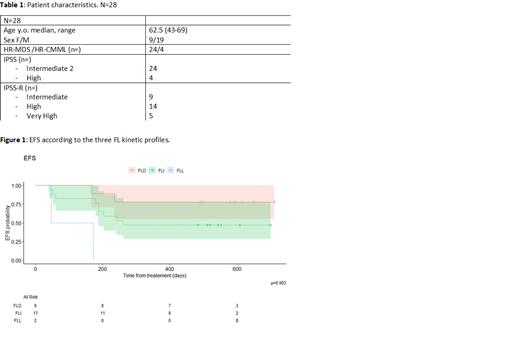
Median (range) FL levels were 4.5 [0- 33.5] on D1, 74.1 [4.1-296.6] at D8, 326.6 [47.4-678] at D15 and 381.3 [4.4-800.1] at D22. As in AML (Peterlin et al, 2021), patients could be categorized according to three FLc kinetic profiles: i) sustained increase from D 1 to 22 (FLI group n=17, 61% of the cohort), ii) increase from D 1 to 15, then decrease on D 22 (FLD group, n=9, 32% of the cohort) and iii) persistent low levels (<100 pg/mL from D 1 to 22, FLL group, n=2, 7% of the cohort). Median (range) IL6 levels were 11 (0-587.8) on D1 and 179.8 (0-531) on D22. Increase of IL6 levels between D1 and D22 was observed in 19 patients (68%) and decrease in 7, while 2 patients had no IL6 detectable at either times. Finally, median (range) CRP levels were 2.3 (0-108) on D1 and 40.5 (7.2-323) on D22. Increase of CRP level was observed in most patients between D1 and D22 (n=25, 96%). There was no correlation between IL6 and CRP levels on D1 (p=0.64) or D22 (p=0.48).
CR/CR rates, 1-y OS and EFS were not correlated with FL levels (< vs > median) at the 4 time points considered. However, a FLD kinetic profile was significantly associated with higher CR/CRi rates (100% vs FLI 47% vs FLL 50%, p=0.002) and 1-y EFS (78% (55-100) vs FLI 47% (28-78) vs FLL: 0%, p=0.003), Figure 1). 1-y OS was similar between the 3 FL kinetic profile groups (FLD: 89% (71-100) vs FLI 76% (59-100) vs FLL 50% (13-100), p=0.7)). In multivariate analysis, taking into account age, gender, R-IPSS and the FL kinetic profile during cycle 1 of CPX-351, a FLD kinetic profile was the only factor associated with higher CR/CRi rate (OR: 24.2, 95%CI: 2.4-3278, p=0.004) and better EFS (OR: 21.91; 95%CI: 2.47-194.77, p=0.006). Levels and kinetic profiles of IL6 and CRP had no impact on CR/CRi rates, OS and EFS.
Conclusion: As in AML, FL kinetic profile predicts response and survival in HR-MDS/CMML. A FLD kinetic profile was the only factor independently associated with higher CR/CRi rates and better EFS. These results have to be confirmed on larger cohorts. Other treatment approaches may be of interest in patient with a non FLD profile.
OffLabel Disclosure:
Dumas:Novartis: Honoraria, Other: Research support for institution; Servier: Honoraria, Other: Research support for institution; BMS: Honoraria, Other: Research support for institution; Abbvie: Honoraria; Astellas: Honoraria, Other: Research support for institution; Daiichi-Sankyo: Honoraria, Other: Research support for institution; Jazz pharmaceutical: Honoraria; Janssen: Honoraria; Roche: Other: Research support for institution. Cluzeau:Incyte: Speakers Bureau; Syros: Speakers Bureau; Servier: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Keros: Speakers Bureau; Jazz Pharma: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau. Chevallier:Incyte: Honoraria, Research Funding; Takeda: Honoraria; Immedica Pharma: Honoraria; Mallinckrodt Pharmaceuticals: Honoraria; Sanofi: Honoraria; Servier: Honoraria.
The study that we are presented here (i.e FLAM-MDS study) is a substudy of the GFM-CPX-MDS trial allready published aiming to evaluate cpx-351 in HR-MDS and CMML (Peterlin et al.Lancet Haematology 2023). The FLAM-MDS study was a secondary objective of GFM-CPX-MDS trial aiming to assess the prognostic value of those two cytokines in the same cohort of patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal